Creative Bioarray provides wound healing assays for quantitative analysis of cell proliferation and cell migration. Modified assays can be developed for changeable experimental conditions to meet specific requirements of research and drug development.
Introduction of Wound Healing Assays
Cell migration is an integrated multistep biological process, which is involved in the development and maintenance of muticellular organisms as well as the pathogenesis of various diseases. Wound healing assay, also known as cell scratch assay, is one of the earliest methods developed for the study of directional cell migration in vitro. The common method is to scratch in the cell monolayer and then monitor and quantify the cell migration towards the artificial scratch. This assay can give insight into conditional migration, cell-cell interactions, cell-matrix interactions, cell invasion, angiogenesis and high throughput drug screening.
Wound healing assays have various advantages, such as relatively cheap, simple and straightforward, versatility, possibilities of real-time measurement and high-throughput screening, easily adjustment of the experimental conditions for different experimental objectives, allowing for a strong directional migratory response, etc. In view of this, it is still one of the most widely used methods to study the migration of monolayer cultured cells.
However, typical wound healing assays have one limitation that the depth and size of the scratch could be inconsistencies. This may have a great influence on the accuracy and reliability of the results and findings. In order to solve this problem, several auxiliary tools have been developed, such as culture inserts. Combined with live-cell microscopy, fluorescence-based quantitative methods, automation and high-throughput techniques, and other new technologies, the accuracy and efficiency of wound healing assays can be further improved.
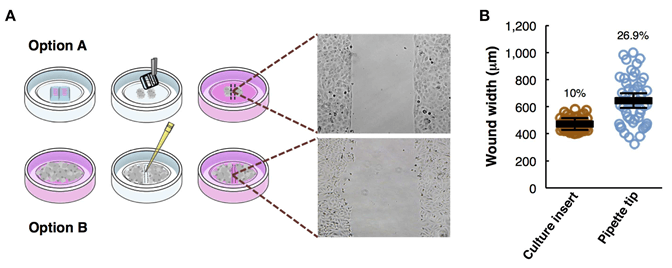 Figure 1. Overview of the wound healing assay preparation protocols. (Pijuan J, et al., 2019)
Figure 1. Overview of the wound healing assay preparation protocols. (Pijuan J, et al., 2019)
Modified Wound Healing Assays Available at Creative Bioarray
Wound healing assays can be modified to study the effects of various changeable experimental conditions. According to different needs, our professional scientific team can provide customized solutions based on the following modified assays.
Fluorescence-Based Wound Healing Assay
Fluorescence detection-based methods, such as live cell staining, can be used to quantitatively evaluate cell migration. It is also possible to track the migration of individual cells by transfection of plasmids with fluorescent groups. These assays offer simplicity, sensitivity, accuracy, and real-time monitoring. Using fluorescent scanner can make it integrated into automated large-scale screening studies.
Wound Healing Assays Using Live-Cell Microscopy
The dynamic process of cell migration can be observed by using live-cell microscope, a common technique for real-time imaging of living cells. Cell imaging system combined with different kinds of microscope, such as fluorescence microscope, confocal microscope and transmitted-light microscope, can achieve different research purposes, from the single-cell level to their function in the whole organism.
High-Throughput Wound Healing Assays
We can adapt the typical wound healing assay to multi-well plate format and mechanically create scratches of specific size. Automated microscopy can be used to monitor cell migration. High-throughput wound healing assays can greatly improve research efficiency and facilitate drug screening.
Automated Wound Healing Assays
Automated scratch creation, kinetic image capture and analysis, and automated computational tools have greatly improved the speed and accuracy of wound healing assays. These automated assays have improved significantly in quality and reliability, making them suitable for drug screening.
Microfluidic Wound Healing Assays
Microfluidic devices can be used to create scratch in wound healing assays by introducing fluid flows. The microfluidic assay is possible to provide a stable gradient of growth factors to the wound. It also offers unique advantages, such as the application of shear stress.
Creative Bioarray provides high-quality services in a timely and cost-effective manner. We aim to accelerate your research and drug development project. If you need more detailed information, please feel free to contact us. We look forward to cooperating with you.
References:
- Pijuan J, et al. In vitro cell migration, invasion and adhesion assays: from cell imaging to data analysis. Frontiers in Cell and Developmental Biology, 2019, 7: 107.
- Yarrow J C, et al. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC biotechnology, 2004, 4(1): 21.
- Jonkman J E N, et al. An introduction to the wound healing assay using live-cell microscopy. Cell adhesion & migration, 2014, 8(5), pp: 440-451.
- Van der Meer A D, et al. A microfluidic wound-healing assay for quantifying endothelial cell migration. American Journal of Physiology-Heart and Circulatory Physiology, 2009, 298(2), pp: H719-H725.
For research use only. Not for any other purpose.

 Figure 1. Overview of the wound healing assay preparation protocols. (Pijuan J, et al., 2019)
Figure 1. Overview of the wound healing assay preparation protocols. (Pijuan J, et al., 2019)
