Endotoxin Detection and Removal Services
Creative Bioarray provides professional endotoxin detection and removal services to help you avoid adverse effects on the reliability of experimental results and safety of biologics and drugs. Our services cover everything from raw materials to final products to help you ensure all your materials used in research and manufacturing applications are endotoxin-free.
About Endotoxin
Endotoxin, also known as lipopolysaccharide (LPS), is a major part of the outer membrane of Gram-negative bacterial cell wall. It can potentially contaminate cell cultures and has adverse effects on biopharmaceutical process and final products. Endotoxin is cytotoxic and can affect cell function. It can interfere with the structure of cell membrane and may lead to morphological changes and membrane damage.
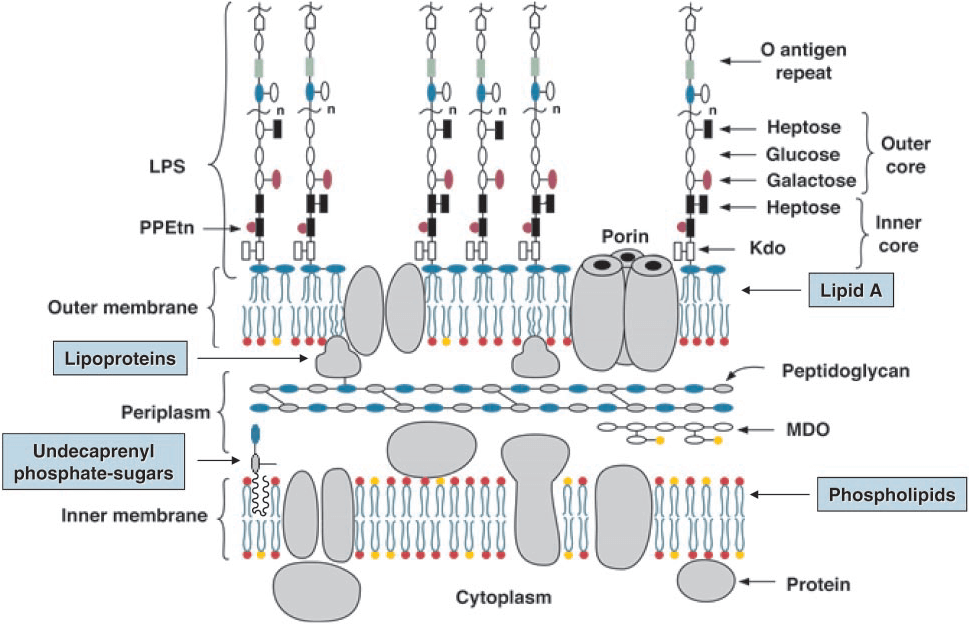 Figure 1. Model of the inner and outer membranes of E. coli K-12. (Raetz, C. R., & Whitfield C, 2002)
Figure 1. Model of the inner and outer membranes of E. coli K-12. (Raetz, C. R., & Whitfield C, 2002)
Endotoxin is heat stable. It can significantly affect the accuracy of both in vitro and in vivo experiments. Endotoxin can also cause severe reactions in humans and animals. It is even related to the pathogenesis of many diseases. Thus, it is very important to monitor the presence of endotoxin in cell culture and downstream products. For biologics and drugs, endotoxin detection and removal are critical quality control procedures.
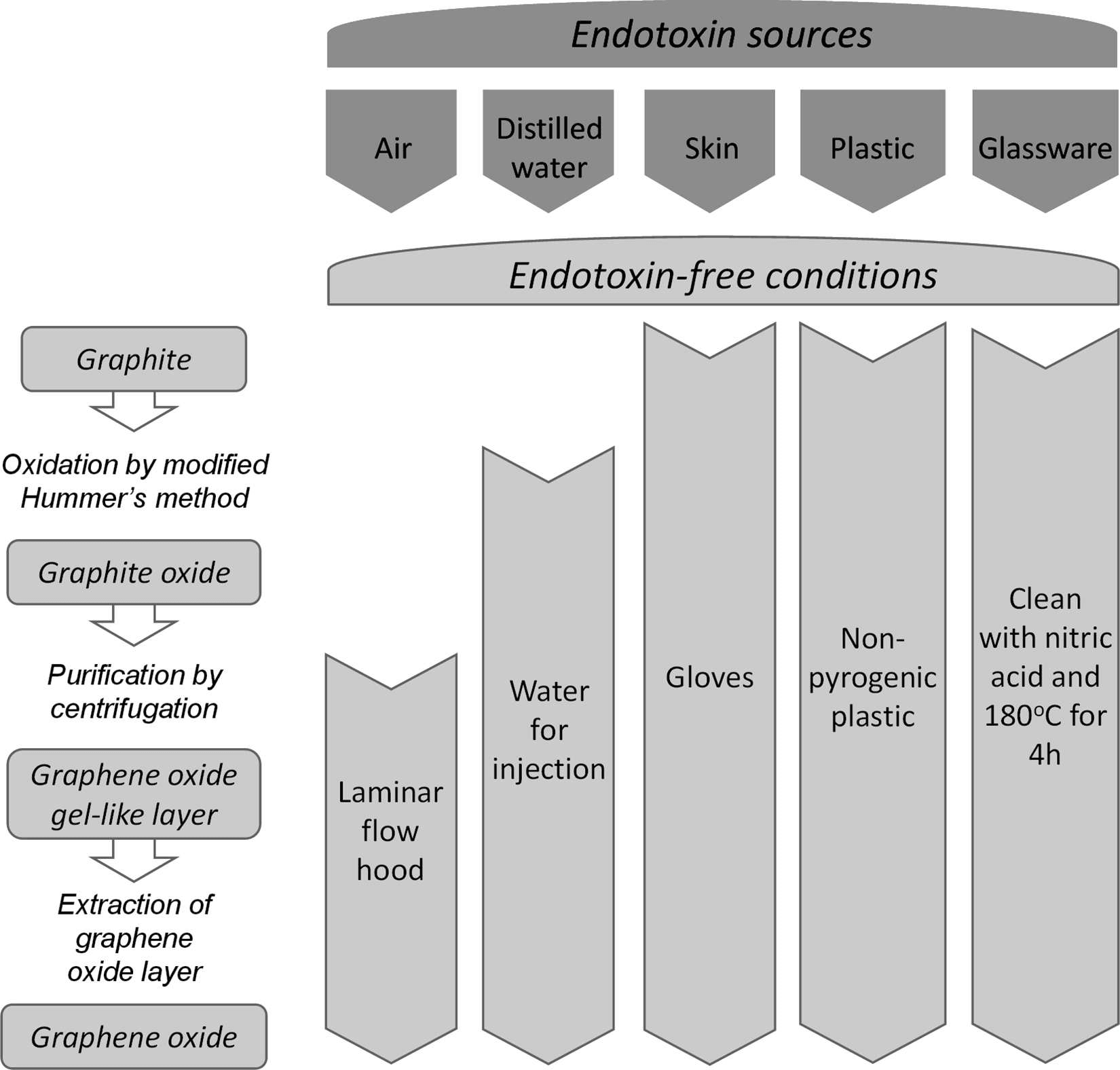
Figure 2. Flow chart depicting the main endotoxin sources identified in the preparation of GO by Hummer's method and guidelines for endotoxin-free conditions for the production of GO samples. (Mukherjee S P, et al., 2016)
Endotoxin Detection Services
Sterility testing can detect living microorganisms, but endotoxin released after bacteria destruction is not detectable. Therefore, it is necessary to detect endotoxin independently. The traditional methods for endotoxin detection are the in vitro Limulus Amebocyte Lysate (LAL) assay, monocyte activation test (MAT) and the in vivo rabbit pyrogen test (RPT).
Our experienced scientists and developing technique platform enable us to carry our rapid endotoxin detection with accurate and reliable results. We can also customize solutions according to the specific needs of our customers. If the detection result is positive, we can continue to provide endotoxin removal services for you.
Endotoxin Removal Services
Endotoxin removal is mainly based on the chemical properties or the molecular weight of endotoxin. There are four common used methods to remove endotoxin from aqueous solutions, including ultrafiltration, activated charcoal and asbestos adsorption, affinity chromatograph and ion exchange chromatography.
We can provide endotoxin removal services for a wide range of materials, such as cell cultures, cell extracts, buffers, reagents, and antibodies. Before and after endotoxin removal, we will carry out quantitative endotoxin detection assays to ensure that endotoxin is completely removed. Our scientific team can also develop customized solutions based on the different properties of target samples to ensure the recovery rate and final application of the samples.
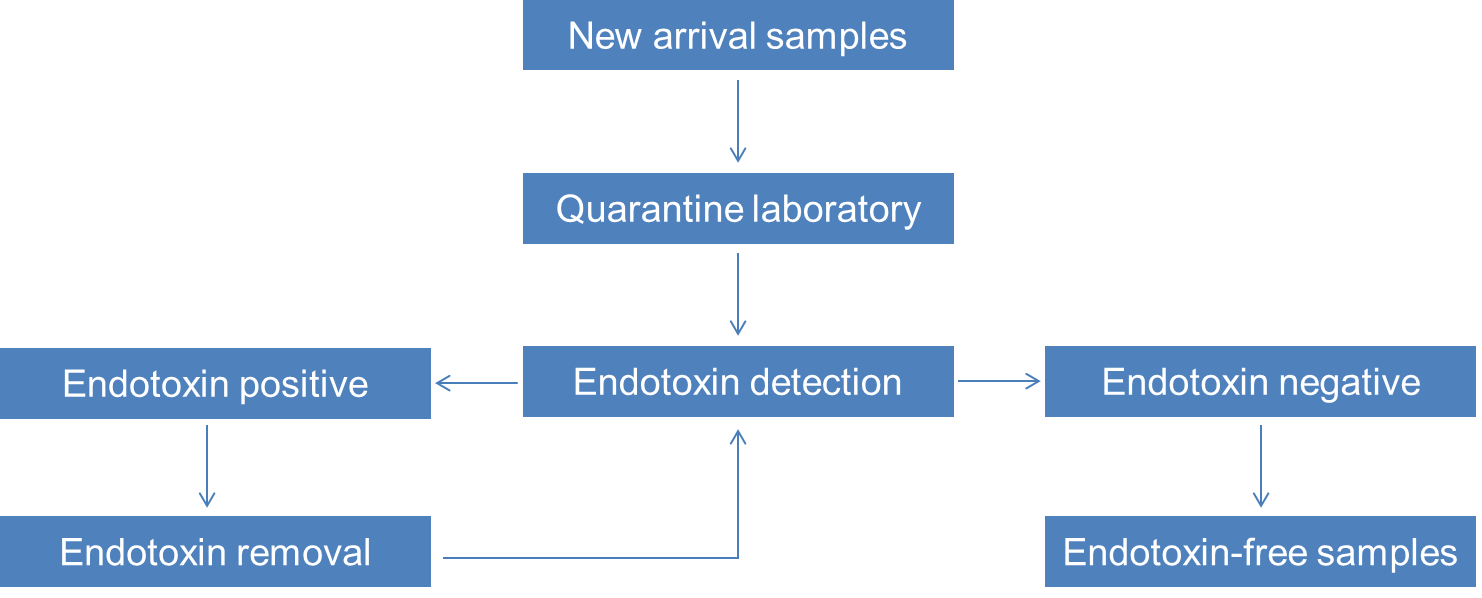
Figure 3. Endotoxin detection and removal services flow chart.
Our Advantages
- Broad application range.
- High sensitive detection and maximum removal rate.
- Accurate and reliable results.
- Excellent sample recovery rate.
- Strict quality control.
- Fast turnaround time and competitive price.
- Customized solutions.
Creative Bioarray offers innovative and efficient technique for endotoxin detection and removal. Our scientific team can tailor the most suitable solution for your specific requirements. If you are interested in our services or have any questions, please feel free to contact us. We look forward to working with you in the near future.
References:
- Raetz, C. R., & Whitfield C. Lipopolysaccharide endotoxins. Annual review of biochemistry, 2002, 71(1), pp: 635-700.
- Mukherjee S P, et al. Detection of endotoxin contamination of graphene based materials using the TNF-α expression test and guidelines for endotoxin-free graphene oxide production. PloS one, 2016, 11(11): e0166816.
For research use only. Not for any other purpose.

 Figure 1. Model of the inner and outer membranes of E. coli K-12. (Raetz, C. R., & Whitfield C, 2002)
Figure 1. Model of the inner and outer membranes of E. coli K-12. (Raetz, C. R., & Whitfield C, 2002) 

