Custom Media Formulation Development Services
Optimized cell culture media are essential for achieving robust cell growth, high productivity, and consistent product quality. Our Media Formulation Development Services offer a fully customized and systematic approach to creating high-performance media designed for your specific cell line, workflow, and production goals.
We provide end-to-end development support—from initial assessment and prototype formulation to optimization, scale-up, and final manufacturing guidance—ensuring your project advances with speed, efficiency, and regulatory confidence.
Services
Customized Media Formulation Development
We specialize in developing chemically defined, animal-component-free (ACF) media for a wide array of production cell lines (e.g., CHO, HEK293, Vero, Insect Cells, Hybridoma, and immune cells for CGT).
- Proprietary Formulation Libraries: Fast identification of the best nutrient combination through our large proprietary database of raw materials and formulations.
- Specialized Experimental Design: Robust and scalable formulation through Design of Experiment and statistical analysis.
- High Throughput Screening (HTS): Screening hundreds of formulations in the shortest time using our high-throughput screening platforms.
- Optimization for high performance: Targeted optimization for improved performance (target protein expression up to 14g/L CHO fed-batch, cell viability, etc.) and consistent product quality.
Process-Specific Optimization
Our service is tailored to your specific bioprocess needs, supporting different modes of culture:
- Fed-Batch and Perfusion: Formulation of basal media and concentrated feeds for long-term, high-density culture.
- Transient Transfection Systems: Media and feeds for transient transfection for high-titer virus (AAV, Lentivirus) and protein production in 293 and insect cells.
- Cell and Gene Therapy (CGT): Custom, clinical-grade media formulation for immune cells (CAR-T, NK) and viral vectors expansion and manufacture.
Seamless Scaling and Manufacturing
We ensure a smooth transition from lab-scale development to commercial-scale manufacturing with a focus on quality and consistency.
- Pilot Batch Production: Production of pilot scale batches to validate performance and manufacturing parameters before scale-up.
- Quality Management System: Quality control system and manufacture under GMP in a dedicated facility with state-of-the-art cGMP pilot and manufacturing units (10,000m2 factory).
- Flexible production & OEM: Custom media manufacturing, or OEM production of classical or client specified formulations with full traceability and documentation.
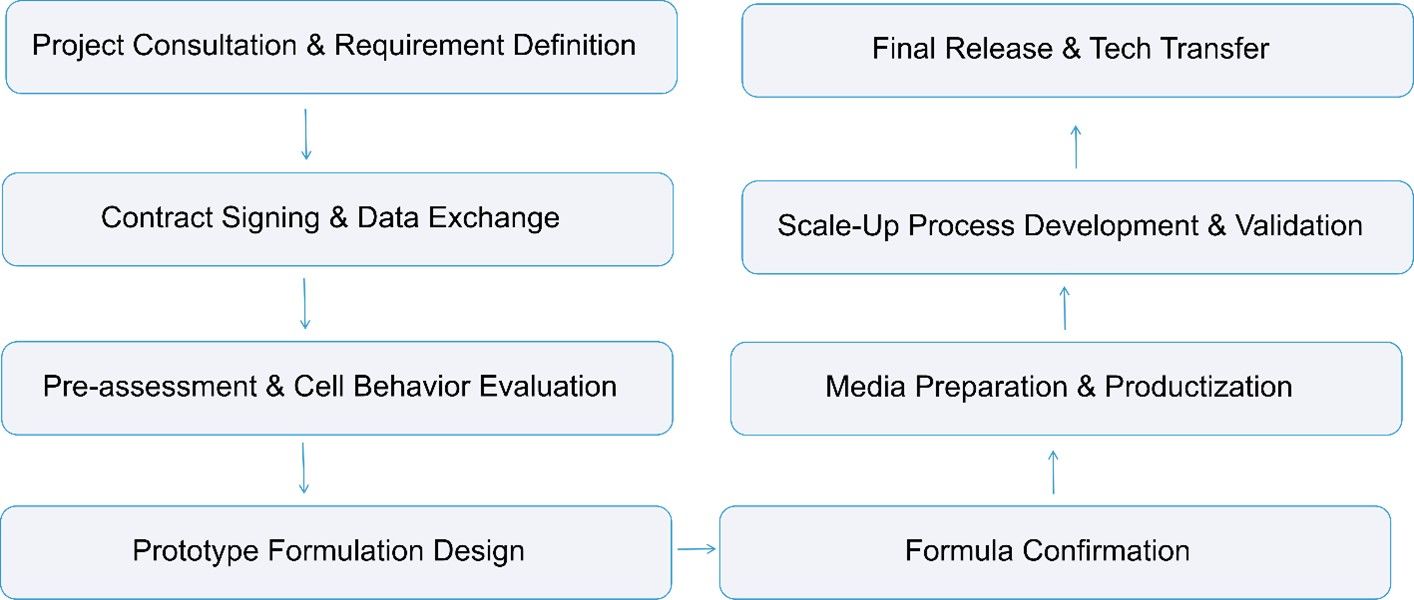
Our Media Formulation Development Process
Why Partner with Us
- Deep Formulation Expertise: Strong know-how across diverse cell systems and bioproduction modes.
- Data-Driven Precision: Advanced analytics and DoE for efficient optimization.
- Customizable & Scalable: Solutions built to transition smoothly from R&D to GMP manufacturing.
- Quality & Reliability: High standards in raw materials, testing, and process evaluation.
For research use only. Not for any other purpose.


